|
|
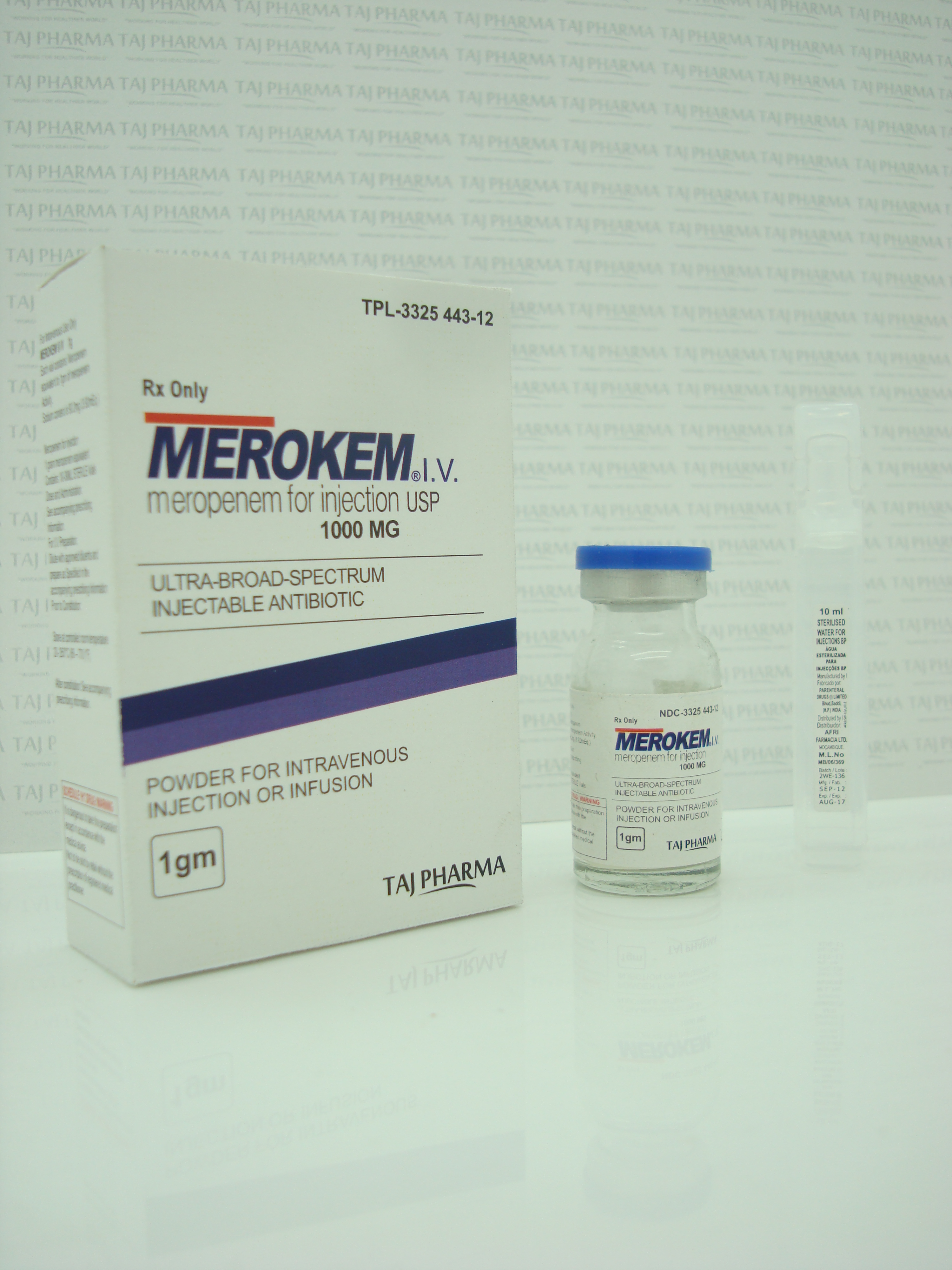
Merokem I.V.
(meropenem) for Injection
FOR INTRAVENOUS USE ONLY
To reduce the development of drug-resistant bacteria and
maintain the effectiveness of Merokem I.V. (meropenem for
injection) and other antibacterial drugs, MERREM I.V. should be
used only to treat or prevent infections that are proven or
strongly suspected to be caused by bacteria.
Each 500 mg MERREM I.V. vial will deliver 500 mg meropenem and
45.1 mg of sodium as sodium carbonate (1.96 mEq).
INDICATIONS
To reduce the development of drug-resistant bacteria and maintain
the effectiveness of MERREM I.V. and other antibacterial drugs,
MERREM I.V. should only be used to treat or prevent infections
that are proven or strongly suspected to be caused by susceptible
bacteria. When culture and susceptibility information are
available, they should be considered in selecting or modifying
antibacterial therapy. In the absence of such data, local
epidemiology and susceptibility patterns may contribute to the
empiric selection of therapy.
MERREM I.V. is indicated as single agent therapy for the treatment
of the following infections when caused by susceptible isolates of
the designated microorganisms:
Skin and Skin Structure Infections
Complicated skin and skin structure infections due to
Staphylococcus aureus (β-lactamase and non-β- lactamase producing,
methicillin susceptible isolates only), Streptococcus pyogenes,
Streptococcus agalactiae, viridans group streptococci,
Enterococcus faecalis (excluding vancomycin-resistant isolates),
Pseudomonas aeruginosa, Escherichia coli, Proteus mirabilis,
Bacteroides fragilis, and Peptostreptococcus species.
Intra-abdominal Infections
Complicated appendicitis and peritonitis caused by viridans group
streptococci, Escherichia coli, Klebsiella pneumoniae, Pseudomonas
aeruginosa, Bacteroides fragilis, B. thetaiotaomicron, and
Peptostreptococcus species.
MERREM I.V. has been found to be effective in eliminating
concurrent bacteremia in association with bacterial
meningitis.MERREM I.V. is useful as presumptive therapy in the
indicated condition (i.e., intra-abdominal infections) prior to
the identification of the causative organisms because of its broad
spectrum of bactericidal activity.
DOSAGE AND ADMINISTRATION
Adults
The recommended dose of MERREM I.V. is 500 mg given every 8 hours
for skin and skin structure infections and 1 g given every 8 hours
for intra-abdominal infections. MERREM I.V. should be administered
by intravenous infusion over approximately 15 to 30 minutes. Doses
of 1 g may also be administered as an intravenous bolus injection
(5 to 20 mL) over approximately 3-5 minutes.
Use in Elderly Patients
No dosage adjustment is required for elderly patients with
creatinine clearance values above 50 mL/min.
Use in Pediatric Patients
For pediatric patients from 3 months of age and older, the MERREM
I.V. dose is 10, 20 or 40 mg/kg every 8 hours (maximum dose is 2 g
every 8 hours), depending on the type of infection (complicated
skin and skin structure, intra-abdominal or meningitis). (See
dosing table below.) Pediatric patients weighing over 50 kg should
be administered MERREM I.V. at a dose of 500 mg every 8 hours for
complicated skin and skin structure infections, 1 g every 8 hours
for intra-abdominal infections and 2 g every 8 hours for
meningitis. MERREM I.V. should be given as intravenous infusion
over approximately 15 to 30 minutes or as an intravenous bolus
injection (5 to 20 mL) over approximately 3-5 minutes.
WARNINGS
SERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (ANAPHYLACTIC)
REACTIONS HAVE BEEN REPORTED IN PATIENTS RECEIVING THERAPY WITH β-
LACTAMS. THESE REACTIONS ARE MORE LIKELY TO OCCUR IN INDIVIDUALS
WITH A HISTORY OF SENSITIVITY TO MULTIPLE ALLERGENS.
THERE HAVE BEEN REPORTS OF INDIVIDUALS WITH A HISTORY OF
PENICILLIN HYPERSENSITIVITY WHO HAVE EXPERIENCED SEVERE
HYPERSENSITIVITY REACTIONS WHEN TREATED WITH ANOTHER β-LACTAM.
BEFORE INITIATING THERAPY WITH MERREM I.V., CAREFUL INQUIRY SHOULD
BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO
PENICILLINS, CEPHALOSPORINS, OTHER β-LACTAMS, AND OTHER ALLERGENS.
IF AN ALLERGIC REACTION TO MERREM I.V. OCCURS, DISCONTINUE THE
DRUG IMMEDIATELY. SERIOUS ANAPHYLACTIC REACTIONS REQUIRE IMMEDIATE
EMERGENCY TREATMENT WITH EPINEPHRINE, OXYGEN, INTRAVENOUS
STEROIDS, AND AIRWAY MANAGEMENT, INCLUDING INTUBATION. OTHER
THERAPY MAY ALSO BE ADMINISTERED AS INDICATED.
CONTRAINDICATIONS
MERREM I.V. is contraindicated in patients with known
hypersensitivity to any component of this product or to other
drugs in the same class or in patients who have demonstrated
anaphylactic reactions to β-lactams.
Presentation
Stability in Plastic Syringes, Tubing and Intravenous Infusion
Sets:
Solutions of MEROKEM I.V. (MEROKEM I.V. concentrations ranging
from 1 to 20 mg/mL) in
Water for Injection or Sodium Chloride Injection 0.9% (for up to
4 hours) or in Dextrose Injection 5.0% (for up to 2 hours) at
controlled room temperatures 15-25°C (59-77°F) are stable in
plastic tubing and volume control devices of common intravenous
infusion sets.
Solutions of MEROKEM I.V.
(MEROKEM I.V. concentrations ranging from 1 to 20 mg/mL) in
Water for Injection or Sodium Chloride Injection 0.9% (for up to
48 hours) or in Dextrose Injection 5% (for up to 6 hours)
are stable at 4ºC (39ºF) in plastic syringes.
NOTE: Parenteral drug products should be inspected visually for
particulate matter and discoloration prior to administration,
whenever solution and container permit.
HOW SUPPLIED MEROKEM I.V. is supplied in 20 mL and 30 mL
injection vials containing sufficient meropenem to deliver 500
mg or 1 g for intravenous administration, respectively.
The dry powder should be stored at controlled room temperature
20-25ºC (68-77ºF) [see USP].
500 mg Injection Vial
1 g Injection Vial
MEROKEM I.V. POWDER FOR IV
INJECTION & INFUSION 500MG\
MEROKEM I.V. POWDER FOR IV INFUSION & INFUSION 1000MG
NAME AND STRENGTH OF ACTIVE SUBSTANCE(S)
MEROPENEM TRIHYDRATE…MG EQUIVALENT TO 500MG MEROPENEM
MEROPENEM TRIHYDRATE…MG EQUIVALENT TO 1000MG MEROPENEM

|
|