|
Humogen is available by prescription only.
Each
Vial Contains:
SOMATROPIN - INJECTION
Humogen® [somatropin [rDNA origin) for
injection
Treatment To Promote
Adequate Growth
HUMOGEN® (SOMATROPIN
FOR INJECTION) IS USED TO TREAT:
• CHILDREN WHO DO NOT MAKE ENOUGH GROWTH HORMONE ON THEIR OWN,
HAVE SHORT STATURE ASSOCIATED WITH TURNER SYNDROME, OR HAVE
SHOX DEFICIENCY; HAVE IDIOPATHIC SHORT STATURE, WHICH MEANS THEY
ARE SHORTER THAN 98.8% OF OTHER CHILDREN OF THE SAME AGE AND
SEX, ARE GROWING AT A RATE NOT LIKELY TO ALLOW THEM TO REACH
NORMAL ADULT HEIGHT, AND FOR WHOM NO OTHER CAUSE OF SHORT
STATURE CAN BE FOUND; WERE BORN SMALLER THAN NORMAL FOR THE
NUMBER OF WEEKS OF PREGNANCY AND WHO DO NOT CATCH UP IN HEIGHT
BY 2 TO 4 YEARS OF AGE
• ADULTS WHO HAVE GROWTH HORMONE DEFICIENCY THAT BEGAN EITHER IN
ADULTHOOD (AS A RESULT OF PITUITARY DISEASE, HYPOTHALAMIC
DISEASE, SURGERY, RADIATION THERAPY, OR TRAUMA) OR IN CHILDHOOD.
PATIENTS TREATED FOR GROWTH HORMONE DEFICIENCY IN CHILDHOOD
WHOSE BONES HAVE STOPPED GROWING SHOULD BE REEVALUATED TO
DETERMINE IF THEY SHOULD CONTINUE GROWTH HORMONE
INDICATION
WHAT IS HUMOGEN® (SOMATROPIN) FOR INJECTION?
HUMOGEN IS A PRESCRIPTION MEDICINE THAT IS USED TO TREAT
GROWTH HORMONE DEFICIENCY (GHD) IN:
1- CHILDREN WITH GROWTH FAILURE WHO PRODUCE LOW AMOUNTS OF
GROWTH HORMONE.
2- ADULTS WITH GHD THAT STARTED AS A CHILD OR AS AN ADULT.
HUMOGEN IS AN INJECTABLE FORM OF A
PROTEIN CALLED GROWTH HORMONE THAT IS PRODUCED BY YOUR BODY.
Important Safety Information for Humogen
What is the most important information I should know about
Humogen?
• Do not take Humogen if you are having serious complications
after having open heart surgery, abdominal surgery, or serious
injuries involving many body systems, or are having
life-threatening breathing problems. Deaths have been reported
in such cases.
• Do not use Humogen in children with Prader-Willi syndrome who
are severely obese or have a history of blocked upper airways or
other severe breathing problems, or sleep apnea. Deaths have
been reported in such cases. Humogen is not approved for the
treatment of patients with Prader-Willi syndrome.
• Do not use Humogen if you have active cancer. Growth hormone
deficiency can be an early sign of some tumors in the brain or
pituitary gland. The presence of these types of tumors should be
ruled out by your doctor before you start Humogen.
• Serious allergic reactions have been reported with Humogen.
Humogen is contraindicated if you know you have allergies to
growth hormone or any of its ingredients. Tell your doctor if
you have an allergic reaction. Do not mix Humogen with the
supplied diluent if you are allergic to metacresol or glycerin.
• Your doctor should check your blood sugar regularly while you
are taking Humogen, especially if you have diabetes,
pre-diabetes, or risk factors for diabetes. New cases of type 2
diabetes have been reported in patients taking Humogen.
• Tell your doctor if you have any visual changes accompanied by
headache, nausea, and/or vomiting while taking Humogen. This may
be a sign of increased pressure in the brain.
• Adults may retain water during Humogen treatment. This may be
brief and may increase with higher doses of Humogen.
• If you have hypoadrenalism and are on glucocorticoid
replacement therapy, your doctor may increase your dosage when
you initiate growth hormone treatment.
• Your doctor should test your thyroid function periodically
during Humogen therapy. Thyroid hormone treatment may need to be
started or adjusted.
• Fracture in the ball of the hip joint can occur in children
who have endocrine problems and in children who have rapid
growth. Any child taking Humogen who develops a limp or
complains of hip or knee pain should be seen by a doctor to
check for this.
• Progression of curvature of the spine (scoliosis) can occur in
children who have rapid growth. Humogen has not been shown to
increase the occurrence of this condition. If the child has
scoliosis, the doctor should carefully monitor the progression
of the scoliosis during Humogen treatment.
• Cases of pancreatitis (inflammation of the pancreas) have been
reported rarely in children and adults receiving growth hormone.
Consult a doctor if you develop abdominal pain while taking
Humogen.
• You should rotate your injection sites to avoid breakdown of
skin and fat. Seek prompt medical attention for any allergic
reaction you experience to the injection of Humogen.
Who should not take Humogen?
Humogen should not be used by:
• People with serious complications after having open heart
surgery, abdominal surgery, serious injuries involving many body
systems, or with life-threatening breathing problems
• Children with Prader-Willi syndrome who are severely obese or
have a history of severe breathing problems
• People with active cancer
• People who have had an allergic reaction to growth hormone
• People with diabetic disease of the retina (the lining in the
back of the eyeball)
• Children who have closed growth plates in their bones
USES :
Various brands of this medication are used for the treatment of
one of the following medical conditions: growth failure, growth
hormone deficiency, intestinal disorder (short bowel syndrome) or
HIV-related weight loss or wasting
HOW TO USE: >>
This medication is given by injection into
a muscle or under the skin. The way you inject this medicine will
depend on the brand that you are using. Check with your pharmacist
to ensure that the way you are injecting your medicine is correct.
It is important to change the location of the injection site to
avoid problem areas under the skin. For best results, this
medication must be used exactly as prescribed by your doctor. It
is important to understand your therapy and to follow your
doctor's instructions closely.
If this medicine is used for
short bowel syndrome, consult your doctor if a special diet (high
carbohydrate/lowfat) or the use of nutritional supplements may be
helpful. If this medicine is used for weight loss/muscle wasting,
it may take up to 2 weeks to notice the effects of the drug. Do
not use more of this medication than prescribed or use it more
often since the risk of side effects will be increased. Learn how
to prepare, mix, and inject your medicine correctly. Be sure to
ask your doctor or pharmacist any questions you may have and
follow the instructions for mixing provided with the medication.
Do not shake while mixing the
solution. Shaking makes the medication not work properly. Before
using, check this product visually for particles or discoloration.
If either is present, do not use the liquid. Needles and syringes
are for one-time use only. Do not reuse. Throw away used needles,
syringes, and medical supplies properly. Consult your pharmacist.
SIDE EFFECTS :
>> Headache, nausea, vomiting, fatigue, muscle
pain, or weakness may occur. If these symptoms continue or become
bothersome, inform your doctor or pharmacist promptly. Tell your
doctor immediately if any of these unlikely but serious side
effects occur: development of a limp, joint/hip/knee pain, unusual
increase in thirst or urination, swelling of hands, ankles or
legs, rapid heartbeat.
Tell your doctor immediately if
any of these highly unlikely but very serious side effects occur:
change in the appearance or size of any mole, severe stomach pain,
vision problems or changes, seizure. Rare (possibly fatal)
lung/breathing problems may be caused by this medication in
children with Prader-Willi syndrome. Those at higher risk include
males, severely overweight children, or those with serious
lung/breathing problems ( e.g., sleep apnea, lung infections, lung
disease). Children should be checked for certain breathing
problems (upper airway obstruction) before and during treatment.
Heavy snoring or irregular breathing during sleep (sleep apnea)
are signs of airway obstruction. Tell the doctor immediately if
these signs occur. Also report any signs of lung infection, such
as fever, persistent cough, or trouble breathing.
A serious allergic reaction to
this drug in unlikely, but seek immediate medical attention if it
occurs. Symptoms of a serious allergic reaction include: rash,
itching, severe swelling, dizziness, trouble breathing. If you
notice other effects not listed above, contact your doctor or
pharmacist.
PRECAUTIONS :>>
Before taking somatropin, tell your doctor or pharmacist if you
are allergic to it; or if you have any other allergies. This
medication should not be used if you have certain medical
conditions. Before using this medicine, consult your doctor or
pharmacist if you have: major surgery or trauma, severe breathing
problems (acute respiratory failure), undergoing therapy for
tumors (cancer), Prader-Willi syndrome (see Side Effects section
above), normal growth has stopped (closed epiphyses). Before using
this medication, tell your doctor or pharmacist your medical
history, especially of: diabetes, thyroid problems, back problems
(scoliosis).
When this medication is given to
newborns, mix with sterile water for injection that does not
contain a preservative. A preservative (benzyl alcohol) which may
be found in the liquid used to mix this product can infrequently
cause serious problems (sometimes death), if given by injection to
an infant during the first months of life. The risk is greater
with lower birth weight infants and is greater with increased
amounts of benzyl alcohol. Symptoms include sudden gasping, low
blood pressure, or a very slow heartbeat. Report these symptoms to
the doctor immediately should they occur. Caution is advised when
using in the elderly because elderly patients may be more
sensitive to its effects.
Needle Instructions
Humogen® [somatropin (rDNA origin) for injection] is injected
under the skin each day, usually at bedtime. This type of
injection is simpler and less penetrating than injections that are
given directly to the muscle or a vein.
Download the Injection Instructions
to obtain your treatment guides to Humogen®.
How to Inject Humogen®
Before each injection, gather the tools and place them on a clean,
flat surface and in an area that has good light. Wash hands with
soap and water thoroughly before beginning. If someone else is
administering the injection, make sure they put on a pair of
disposable gloves.
IMPORTANT:
>> Be sure all injection items are unopened prior
to use. If you find an open needle or syringe packet, do not use
it. Dispose of it in a sharps container. Needles and syringes
should never be shared or reused.
Injecting Humogen® [somatropin (rDNA origin) for
injection]
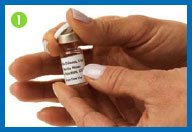
1. Remove the flip tops from the
drug and diluent vials, and throw them away.
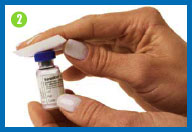
2. Clean both rubber
stoppers with a fresh alcohol swab, then throw the swab away.
Do not touch the vial tops with your hands or gloves after
cleaning them. If you do, wipe the tops again with a fresh alcohol
swab.
3. Prepare your
needle.
Unwrap the syringe and the larger needle without
touching the hub of the needle where it connects to the syringe.
Remove the plastic tip protector from the syringe.
Keeping the needle cap, attach it securely to
the syringe.
If you touch the hub of the
needle or the bottom of the syringe, dispose of them in your
sharps container and use new ones.
4.
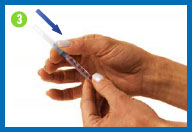
Prepare the
syringe
Pull back on the syringe plunger
until it reaches the 1cc/1ml point on the syringe barrel, or the
volume indicated by your healthcare provider.
While holding the syringe in one
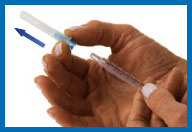
hand, pull the needle cap straight off with the other hand. Set
the needle cap aside for use later.
If the needle and the syringe
come apart — and they have not touched anything — simply put them
back together again. If the needle and/or syringe have touched
anything, dispose of them in your sharps container, and start over
again from Step 3.
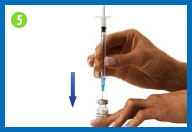
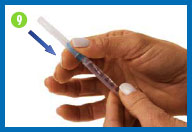
5. Fill syringe with diluent
Place the vial of diluent on
your clean work surface.
Carefully insert the needle
straight down through the center of the rubber stopper into the
vial of diluent.
Gently inject all the air from
the syringe into the vial by pushing down on the plunger. (The
injected air will make it easier to withdraw the solution).
Without removing the needle,
hold the plunger with one hand and turn the vial upside down. Pull
back the syringe to make sure that the needle tip is below the
diluent level in the vial.
Pull the plunger to allow the
diluent to fill the barrel of the syringe to the appropriate
level.
When you have the right amount
of diluent in the syringe, remove the needle from the vial being
careful not to touch the needle or allow it to touch any surface.
Throw the vial of diluent away.
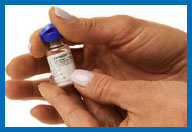
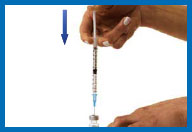
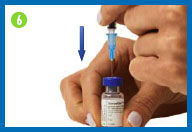
6. Prepare your Humogen®
[somatropin (rDNA origin) for injection]
for injection
Place the vial containing Humogen® powder on
your work surface.
Insert the needle through the
center of the rubber stopper into the Humogen® vial. Slowly and
gently push the plunger, allowing the diluent to flow down the
side of the vial. Do not squirt the diluent because this will make
the solution foamy. If it becomes foamy, let it sit until the
bubbles disappear and the liquid becomes clear.
Keeping the needle in the vial, gently swirl the
vial until the powder is dissolved. Do not shake or turn the vial
upside down.
Once the medication has been
mixed, give yourself the injection as indicated in the
instructions in the next steps. Reconstituted solution has to be
used immediately. Any unused solution should be discarded.
Do not inject Humogen® if the product is
cloudy after reconstitution (in this case, contact your healthcare
provider).
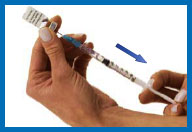
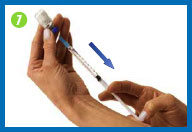
7. Fill the syringe with
the solution
Turn the vial upside down making sure the tip of
the needle is below the fluid level.
Pull back on the plunger and fill the syringe
barrel with the solution.
Make sure that the tip of the needle remains in
the solution while slowly backing the needle out of the vial to
withdraw as much solution as possible.
Carefully remove the needle from the vial
without touching the needle or allowing it to touch any surface.
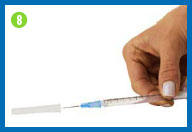
8.
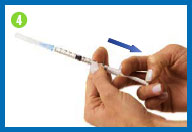
Change the needle
Before you give yourself the
injection, you must replace the larger needle with the smaller
needle.
Take the needle cap you set
aside, place it on your work surface, and recap the needle with a
scooping movement, as indicated in the photo. The cap should snap
down on the needle. Do not touch the needle or allow it to touch
any surface.
Once the cap is on the needle,
gently twist the needle cap off of the syringe and place them in
your sharps container.
Do not squirt any of the
solution out of the syringe.
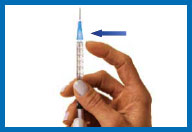
9. Put on the smaller needle
Unwrap the smaller needle and twist it onto the syringe,
then carefully remove the needle cap.
Hold the syringe straight with the needle facing
up.
To remove any air from the syringe barrel and
the needle, gently tap the side of the syringe with your
fingertips until all the bubbles float to the top of the syringe.
Then gently push the plunger until a small drop of liquid appears
on the tip of the needle.
10. Pick an injection site
Find a place on your body where it will be easy for you to give
yourself the injection. Recommended areas include the top of the
thigh and either side of the navel. Make sure to change the
location of your injection each day so that one area does not get
injected too many times.
Before you inject, take an alcohol swab and
carefully clean a four-inch square area around your injection
site. Throw the swab away and let the skin dry.
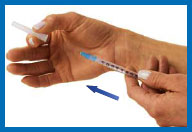
11. Inject Humogen® [somatropin
(rDNA origin) for injection]
Hold the syringe in one hand and remove the cap from the needle.
With your other hand, gently pinch a three-inch
fold of skin.
Point the needle downward, with the slanted,
cutting edge of the needle facing up, about an inch away from the
area you plan to inject.
Push the needle tip into the pinched skin as far
as it will go. Hold the syringe steady and let go of the skin.
Push the plunger down slowly and gently as far
as it will go.
When the syringe is empty, pull the needle
straight out of the skin and put it in your sharps container.
If you accidentally stick yourself with the
needle after your injection, clean the stuck area with soap and
water to prevent infection. Place a 2"x 2" gauze patch over the
area and apply gentle pressure to stop any bleeding.
If someone else gets pricked by the needle after
the injection, let the area bleed freely. Then wash the area
thoroughly with soap and water, and call their doctor immediately
to explain what happened.
12.
Clean up after
injecting
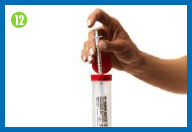
Immediately discard the injection needle, needle
cap, syringe, used gauze patches, and used gloves in your sharps
container. |