
Alfuzosin
CAS number 81403-80-7
Identifiers
ATC code G04CA01
PubChem 2092
DrugBank APRD00490
ChemSpider 2008
Chemical data
Formula C19H27N5O4
Molecular Weight: 425.91
Pharmacokinetic data
Bioavailability 49%
Protein binding 82%-90%
Metabolism Hepatic (CYP3A4-mediated)
Half life 10 hours
Excretion Fecal (69%) and renal (24%)
What is alfuzosin?
Alfuzosin is in a group of drugs called alpha-adrenergic blockers.
Alfuzosin helps relax the muscles in the prostate and bladder neck,
making it easier to urinate.Alfuzosin is used to treat benign prostatic
hyperplasia (enlarged prostate).Alfuzosin may also be used for other
purposes not listed in this medication guide.
Alfuzosin Dosing Information
Usual Adult Dose for Benign Prostatic Hyperplasia:
10 mg orally once a day immediately after
the same meal each day.
DRUG DESCRIPTION
Alfuzosin hydrochloride is a white to
off-white crystalline powder that melts at approximately 240°C. It is
freely soluble in water, sparingly soluble in alcohol, and practically
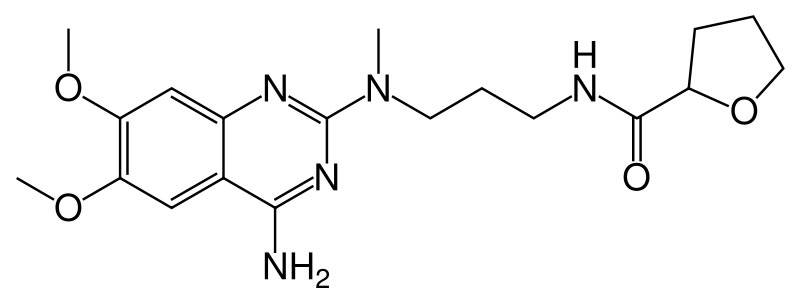
insoluble in dichloromethane. Alfuzosin hydrochloride is (R,S)-N-[3-[(4-amino-6,7-dimethoxy-2-quinazolinyl)
methylamino] propyl] tetrahydro-2-furancarboxamide hydrochloride. The
empirical formula of alfuzosin hydrochloride is C19H27N5O4•HCl.
DIN (Drug Identification Number)
02315866 APO-ALFUZOSIN 10MG TABLET
Chemical Data
Alfuzosin Systematic (IUPAC) name
N-[3-[(4-amino-6,7-dimethoxy-quinazolin-2-yl)- methyl-amino]propyl]
tetrahydrofuran- 2-carboxamide Identifiers CAS number 81403-80-7 ATC
code G04CA01 PubChem 2092 DrugBank APRD00490 Chemical data Formula
C19H27N5O4 Mol. weight 389.449 g/mol Pharmacokinetic data
Bioavailability 49% Protein binding 82%-90% Metabolism Hepatic
(CYP3A4-mediated) Half life 10 hours Excretion Fecal (69%) and renal
(24%) Therapeutic con....
Storage
Store Uroxatral at room temperature,
between 68 and 77 degrees F (20 and 25 degrees C). Store away from heat,
moisture, and light. Keep Uroxatral out of the reach of children and
away from pets.
Chemical formulas
C19H30N3O4 · HCl 2H2O,
Alfuzosin hydrochloride is C19H27N5O4•HCl,
Molecular Formula: C6H10ClNO4,
| Information
Associated with Product : |
|
|
|








