|
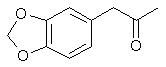
3,4-methylenedioxyphenyl-2-propanone
|
|
IUPAC Name:
1-(1,3-benzodioxol-5-yl)propan-2-one
CAS Registry Number: 4676-39-5
Synonyms: Methyl piperonyl ketone,
5-Acetonyl-1,3-benzodioxole, 3,4-Methylenedioxyphenyl acetone,
3,4-Methylenedioxybenzyl methyl ketone,
1-(1,3-Benzodioxol-5-yl)acetone, CID78407, NSC16688, EINECS
225-128-6, 1-(Acetonyl)-3,4-methylenedioxybenzene, NSC 16688,
ZINC01747237, (3,4-(Methylenedioxy)phenyl)-2-propanone, AI3-30059,
2-Propanone, 1-(1,3-benzodioxol-5-yl)-, 2-Propanone, (3,4-(methylenedioxy)phenyl)-,
2-Propanone, [3,4-(methylenedioxy)phenyl]-, 2-Propanone, 1-[3,4-(methylenedioxy)phenyl]-,
2-Propanone, 1-(1,3-benzodioxol-5-yl)- (9CI), 2-Propanone, 1-(3,4-(methylenedioxy)phenyl)-
(8CI), 4676-39-5
Molecular Formula: C10H10O3 Molecular
Weight: 178.184600 [g/mol]
H-Bond Donor: 0 H-Bond Acceptor: 3
Chemical Name: PIPERONYL METHYL KETONE |
3,4-methylenedioxy-phenyl-2-propanoneis a chemical compound
consisting of phenylacetone substituted with a methylenedioxy functional
group.
It is a chemical precursor of MDA, MDMA (more commonly known as
"Ecstasy" or "XTC"), MDEA and related chemicalsMDP2P is most commonly
synthesized by oxidizing the plant oil safrole or its isomer isosafrole
using the Wacker oxidation or peroxyacid oxidation.
3,4-methylenedioxyphenyl)-2-propanone (MDP-2-P or PMK) was prepared by
two different routes, i.e. by oxidizing isosafrole in an acid medium and
by 1-(3,4-methylenedioxyphenyl)-2-nitropropene reduction.
The final product-MDP-2-P was subjected to GC/MS analysis.
The intermediates and reaction by-products were identified and the
‘route specific’ impurities were established.
3,4-methylenedioxyphenyl)butan-2-amine (MDP-2-MB, MBDB) is a new
homologue of N-methyl-1-(3,4-methylenedioxyphenyl)propan-2-amine (MDMA),
which is strictly controlled as a narcotic.
As part of our continuous survey on illegal designer drugs in the
Japanese market, we found that
N-methyl-4-(3,4-methylenedioxyphenyl)butan-2-amine (MDP-3-MB, HMDMA) was
being sold as MBDB.
As this is the first time that HMDMA has been revealed to be in market
distribution, and its physico-chemical data is thus far unreported, we
describe the structure elucidation of HMDMA and comparative analysis
with related compounds.
The impurity profiles were obtained by means of GC/MS, some reaction
by-products were identified by means of the EI mass spectra including
low energy EI mass spectra and ‘route specific’ impurities were
established. 4-Methyl-5-(3,4-methylenedioxyphenyl)-[1,3]dioxolan-2-one
,N-methyl-2-methoxy-1-methyl-2-(3,4-methylenedioxyphenyl)-ethaneamine,3-methyl-6,7-methylenedioxyisoquinoline-1,4-dione
and N-cyclohexyloacetamide were found to be the synthesis markers of
greatest importance.
Abstract
This paper describes the structural elucidation of a compound produced
during the synthesis of 3,4-methylenedioxymethylamphet-amine (MDMA) via
the reductive amination of 3,4-methylenedioxyphenyl-2-propanone
(3,4-MUP-2-P) with methylamine and sodium cyanoborohydride. The compound
was isolated from MDMA by column chromatography, proton and carbon
nuclear magnetic resonance spectroscopy, LC/mass spectrometry, and total
synthesis were used to identify the compound as N-cyanomethyl-N-methyl-l-(3',4'-methylenedioxyphenyl)-2-propylamine.
This compound has been identified as a potential synthetic route marker
for the reductive amination of 3,4-MDP-2-P with methylamine and sodium
cyanoborohydride and as such it should prove valuable to forensic
scientists engaged in profiling illicit drugs. Profiling MDMA can
provide useful information to law enforcement agencies relating to
synthetic route, precursor chemicals and reagents employed and may be
used for comparative analyses of different drug seizures.
This paper also describes the structural elucidation of the analogous
methylamphetamine synthetic route marker compound,
N-cyanomethyl-N-methyl-l-phenyl-2-propylamine, produced during the
reductive amination of phenyl-2-propanone using methylamine and sodium
cyanoborohydride.
Background
The investigation of clandestine drug manufacturing laboratories
represents a combined effort between the criminal investigator and the
forensic chemist. At an early point in an investigation the special
agent will frequently request a list of the chemicals and synthesis
methods used to produce a controlled substance. Providing these lists is
often a very simple assignment the forensic chemist. A general
understanding of various chemicals reactions and techniques is a part of
the forensic chemist's training, academic background, and experience.
Additionally, numerous specific and detailed drug syntheses are also
available to him from the open literature. The chemist may, none the
less, encounter problems when reviewing published procedures. If the
literature procedures do not explicitly illustrate the synthesis of the
desired pound, the chemist may erroneously assume that it is not
applicable to the clandestine laboratory. This conclusion may, in part,
be due to the complicated nature of the procedure or to the apparent
requirement for specialized equipment. It may also arise from the
failure of the chemist to visualize an application of the literature to
the synthesis of the clandestine drug. In this context, the synthesis
methods are themselves clandestine; they are "hidden" within the
literature. A determined study of literature procedures, however, often
reveals that while they do not detail the synthesis of the drug in
question, they can be modified to give useful or simple methods for its
manufacture. Sometimes this requires only the substitution of
appropriate chemicals or certain changes in reaction parameters or
catalysts.
Examples of this conceptual approach can be shown by the synthesis of
the nonpsychoactive controlled substance phenyl-2-propanone (P-2-P).
Halting the clandestine manufacture of P-2-P is of particular interest
to enforcement personnel since it serves as the primary precursor in a
number of syntheses for amphetamine and methamphetamine. By substitution
of the chemicals and through slight changes in procedure, two published
syntheses have been modified for P-2-P manufacture. These simple changes
are illustrated below and are of the type to be expected of a
clandestine drug chemist. By procuring chemicals and using procedures
not generally recognized for the production of the controlled substance,
the clandestine chemist may improve his chances to escape detection.
Each of the two procedures investigated give fair to excellent yields of
P-2-P, and, by using the procedures consecutively, yields are greatly
increased.
Experimental
Procedure
The following procedure, which Tsuji et. al. [1] used for the
preparation of 1-decanone, required only the substitution of
allylbenzene (1-phenyl-2-propene) for 1- decene. A three-neck round
bottomed flask was fitted with a magnetic stirrer and a
pressure-equalized dropping funnel containing allylbenzene.
The flask was charged with a mixture of palladium chloride, cuprous
chloride, and aqueous N,N-dimethylformamide (DMF). With all outlets
securely stoppered and wired down, an oxygen-filled balloon was placed
over one neck and the flask contents stirred at room temperature to
allow oxygen uptake. After a period of oxygenation, allylbenzene was
added dropwise. The solution was continuously stirred under the
pressurized balloon. During this period of addition, the color of the
solution turned from green to black and gradually returned to green as
the reaction approached completion. The mixture was poured into cold
hydrochloric acid and extracted with methylene chloride (CH2Cl2). The
extract was washed with saturated sodium bicarbonate and dried over
anhydrous sodium sulfate. Through filtration and distillation,
phenyl-2-propanone and trans-beta-methylstyrene (1-phenyl-1-propene)
were recovered.

 Note:
These API/ chemicals are designated as
those that are used in the manufacture of the controlled substances and
are important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your country
government laws /control substance ACT. Note:
These API/ chemicals are designated as
those that are used in the manufacture of the controlled substances and
are important to the manufacture of the substances. For any (Control
Substance) products Import and Export *** subjected to your country
government laws /control substance ACT.
Note /Government Notification:
N/A
|
|