
Oseltamivir Phosphate ( INN)
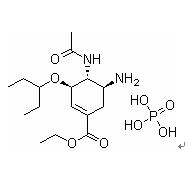
Product name: Oseltamivir phosphate
Chemical name:
Ethyl(3R,4R,5S)-4-acetamido-5-amino-3-pentan-3-yloxycyclohexene-1-carboxylate
phosphate
CAS No.: 204255-11-8
Molecular formula : C16H28N2O4.H3PO4
Assay: 97%min
Product Description
Appearance: White to off-white
crystalline powder
Purity(HPLC): 99%min
Melting Point : 201-203degree
Optical Rotation: -34.0- -38.0degree
Moisture: 0.30%max
Heavy metals: 20ppm max
Residue on ignition: 0.1%max
Impurity Ro-1637: 0.03%max
Any other individual impurity: 0.50%max
Total impurities: 1.0%max
| Productivity: |
500kg/month |
Mode of action
 Oseltamivir
is a neuraminidase inhibitor, serving as a competitive inhibitor towards
sialic acid, found on the surface proteins of normal host cells. By
blocking the activity of the neuraminidase, Oseltamivir prevents new
viral particles from being released by infected cells. Oseltamivir
is a neuraminidase inhibitor, serving as a competitive inhibitor towards
sialic acid, found on the surface proteins of normal host cells. By
blocking the activity of the neuraminidase, Oseltamivir prevents new
viral particles from being released by infected cells.
Resistance
As with other antivirals, resistance to the agent was expected with
widespread use of oseltamivir, though the emergence of resistant viruses
was expected to be less frequent than with amantadine or rimantadine.
The resistance rate reported during clinical trials up to July 2004
was 0.33% in adults, 4.0% in children, and 1.26% overall. Mutations
conferring resistance are single amino acid residue substitutions in the
neuraminidase enzyme.
H3N2

Mutant H3N2 influenza A virus isolates resistant to oseltamivir were
found in 18% of a group of 50 Japanese children treated with
oseltamivir.This rate was similar to another study where resistant
isolates of H1N1 influenza virus were found in 16.3% of another cohort
of Japanese children. Several explanations were proposed by the authors
of the studies for the higher-than-expected resistance rate detected.
First, children typically have a longer infection period, giving a
longer time for resistance to develop. Second, Kiso et al. claim to have
used more rigorous detection techniques than previous studies.
H5N1 avian influenza
High-level resistance has been detected in one girl suffering from
H5N1 avian influenza in Vietnam. She was being treated with
oseltamivir at time of detection. de Jong et al. (2005) describe
resistance development in two more Vietnamese patients suffering from
H5N1, and compare their cases with six others. They suggest that the
emergence of a resistant strain may be associated with a patient's
clinical deterioration. They also note that the recommended dosage of
oseltamivir does not always completely suppress viral replication, a
situation that could favor the emergence of resistant strains. Moscona
(2005) gives a good overview of the resistance issue, and says that
personal stockpiles of Tamiflu could lead to under-dosage and thus the
emergence of resistant strains of H5N1.
Resistance is of concern in the scenario of an influenza pandemic
(Wong and Yuen 2005), and may be more likely to develop in avian
influenza than seasonal influenza due to the potentially longer duration
of infection by novel viruses. Kiso et al. suggest that "a higher
prevalence of resistant viruses should be expected" during a pandemic.
Note: Veterinary use
There have been anecdotal reports of oseltamivir reducing disease
severity and hospitalization time in canine parvovirus infection. The
drug may limit the ability of the virus to invade the crypt cells of the
small intestine and decrease gastrointestinal bacteria colonization and
toxin production.

|





 Oseltamivir
is a neuraminidase inhibitor, serving as a competitive inhibitor towards
sialic acid, found on the surface proteins of normal host cells. By
blocking the activity of the neuraminidase, Oseltamivir prevents new
viral particles from being released by infected cells.
Oseltamivir
is a neuraminidase inhibitor, serving as a competitive inhibitor towards
sialic acid, found on the surface proteins of normal host cells. By
blocking the activity of the neuraminidase, Oseltamivir prevents new
viral particles from being released by infected cells.




